One of the most significant structural changes SB23-290 made to the original Natural Medicine Health Act was the creation of the Natural Medicine Division (“NMD”) within the Colorado Department of Revenue (“DOR”). While the training and licensing of Facilitators remains within the purview of the Division of Professions and Occupations within DORA, the newly-created NMD will oversee all other aspects of the regulated natural medicine program. DOR will set standards and issue licenses for cultivation, production, testing, and healing center facilities. Under SB23-290, DOR must promulgate rules regarding general licensing requirements, qualifications and eligibility requirements for licensure, permitted and prohibited financial interests, testing program requirements, regulation of licensed premises, transportation requirements, production management, and record keeping. DOR may promulgate rules regarding product & packaging requirements, concentration of products, security and surveillance, and health & safety standards.
Initially the NMD consists primarily of four DOR officials who also hold roles within the Department’s Marijuana Enforcement Division (“MED”).
- Heidi Humphrey, State Licensing Authority (Interim DOR Executive Director)
- Dominique Mendiola, Senior Director
- Kyle Lambert, Deputy Senior Director
- Allison Robinette, Director of Policy & Regulatory Affairs
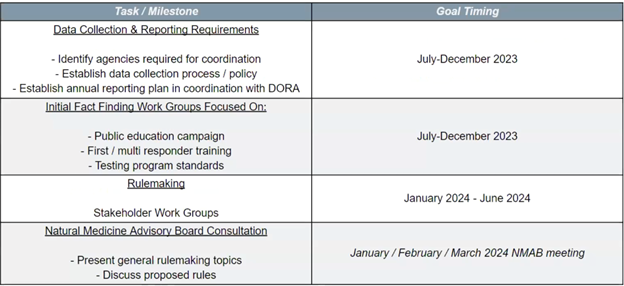
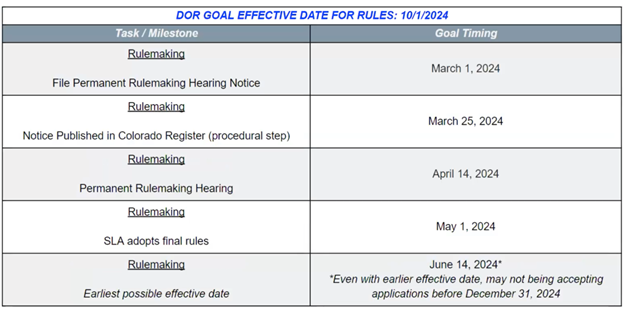
The Department intends to publish permanent rulemaking hearing notices, the first step in the official rulemaking process, in March of 2024. Prior to the start of official rulemaking, the Department will conduct additional listening sessions, collaborate with the NMAB, and create working groups on the subjects of public education, emergency services training, and testing requirements. The Department aims to adopt final rules in May of 2024.
As part of the pre-rulemaking process, the NMD began holding “Listening Sessions” in October with the goal of gathering information from the public at large. The October sessions consisted of five, one-hour, online meetings focused on specific topics announced in advance. Unlike meetings of the NMAB, public attendees of listening sessions are welcome to share their opinions and information and ask questions. Prior to each session, the Department posts discussion questions and a list of related resources to guide the conversation. For new attendees, the Department gives a brief overview of the law generally as well as the specific statutory mandate for the subject of the Listening Session in each session. The sessions then open up for the public to share thoughts, concerns, and information that may be helpful to the Department.
Listening Session #1 – SB23-290 Overview and Introduction to Natural Medicine
The Natural Medicine Division’s first Listening Session consisted primarily of setting the stage for future sessions, followed by a brief Q&A. DOR staff first introduced the Natural Medicine Division by describing the regulated subjects that fall within the Department’s purview, initial staff, statutory funding requirements, and their intent to begin official rulemaking in early 2024.
Ms. Mendiola emphasized the restrictions inherent in the cash-fund nature of the program – primarily the program must be supported by application and licensing fees. To keep initial administrative costs low, the department is relying on staff from the Marijuana Enforcement Division to operate the Natural Medicine Division at its outset. Additional staffing will take an incremental approach, opening and filling roles as needed. The Department will also be looking to leverage existing resources within the Department, collaborate with other state agencies, and utilize lessons and expertise gleaned from the cannabis industry to guide its actions.
Next, NMD staff gave an overview of the framework created by Proposition-122 and SB23-290 including division of responsibilities between state regulatory agencies, personal use provisions, rulemaking timelines, and limitations such as the exclusion of Peyote from regulated and personal use, remuneration when sharing under personal use, and the exclusion of synthetics from the definition of natural medicine.
In addition to licensing and regulating Healing Centers, cultivation, manufacturing, and testing facilities the department is responsible for developing a testing program, data collection, public education campaigns, training materials for first and multi-responders, and annual reporting.
Mandatory rulemaking authority covers general licensing requirements; qualifications and eligibility requirements for licensure; permitted and prohibited financial interests; testing program; regulation of licensed premises; transportation requirements; production management; and record keeping. The Division is also required to consult with the NMAB before promulgating rules. Permissive rulemaking authority exists for product & packaging requirements, concentration of products, security and surveillance, and health & safety standards.
Allison Robinette then gave an overview of the Division’s timeline, covering pre-rulemaking stages as well as the official rulemaking process. DOR intends to set the official process in motion by filing official notices of permanent rulemaking by March 1, 2024.
In the brief Q&A, seven members of the public shared thoughts or asked questions regarding how to get involved in the creation of the program, including additional stakeholders such as the Indigenous community, hypnotherapists, and the broader existing psychedelic community in Colorado. Ms. Mendiola identified public comment during official rulemaking and the Department’s planned working groups as routes for involvement. Sam Bahrami, Senior Advisor at DORA, also spoke about the work of the Indigenous and Religious Use and Outreach subcommittee and the upcoming Federally Recognized Tribes and Indigenous Community working group. One member of the public asked for a clear definition of a microdose.
Listening Session #2: First & Multi-Responder Training Part 1
Following an opening welcome, Dominique Mendiola and Allison Robinette gave a brief overview of the Division’s statutory requirements, specifically C.R.S. § 44-450-202(5) which charges the NMD with developing and promoting training materials for first and multi-responders including law enforcement, emergency medical providers, social services providers, and firefighters.
The following discussion questions were presented:
- Discuss the scope of multi-responders that training materials should target (e.g. Law enforcement, EMS, fire)
- What relevant trainings and educational resources are already available?
- What are the perceived gaps in any existing trainings?
- What format of trainings or resources should the Division focus on? (For example, written materials, webinars, or in-person interactive training)
- What information do first and multi-responders need when they are called to respond to a Healing Center vs. responding to a private residence?
- What Product labeling information is most critical for purposes of Emergency Room / first/multi-responders?
- Through the lens and perspectives of indigenous, religious, and spiritual use of natural medicines: Are there specific types of training / subject-matter that should be incorporated into training materials?
- What data is currently available regarding natural medicine-related hospitalizations and adverse events in Colorado that can inform development of training materials for first/multi-responders?
- What documents/disclosures should be made available to first/multi responders when responding to a Healing Center and private residence?
Around 100 members of the public attended, 14 of whom shared comments, questions, or concerns. The speakers covered a wide range of topics including:
- The need to include information on Serotonin Syndrome.
- Information for veterinary first responders.
- Purging/vomiting as a common aspect of consuming natural medicine.
- Making Natural Medicine available to first responders dealing with trauma.
- The possibility of, and need for information on, various mushroom species appearing outside of the regulated market.
- Concerns about emergency services searching private residences.
- Information available from the Rocky Mountain Poison and Drug Center.
- Concerns about armed responses to emergency calls exacerbating psychedelic crisis.
- Importance of educating first responders on possession limits and when there are or are not grounds for intervention.
- Education for dispatchers as the first level of triage in the emergency response system.
- Utilizing tools and systems already in place as part of Colorado’s behavioral health system.
- Concerns about licensees being targeted by law enforcement after placing emergency calls.
Listening Session #3 – Public Education Campaign Part 1
Following a quick refresher on DOR’s Natural Medicine Division and the natural medicine program, Natural Medicine Division director Dominique Mendiola began by identifying the source of the Department’s obligation in C.R.S. § 44-50-202(h). This subsection assigns responsibility for public education on natural medicine to DOR.
Sam Bourdon and Heather Link-Bergman briefly discussed the fentanyl public awareness campaign currently being developed by CDPHE. They shared how the department is thinking about public education in regards to providing accurate information, identifying the right audiences, engaging those audiences in a meaningful way, and reaching the audiences through novel channels that make sense for them.
Ean Seeb, Special Advisor to the Governor on cannabis, shared lessons and experiences from early cannabis public education campaigns. In particular he emphasized the success of campaigns that provided accurate information on what was permitted regarding cannabis consumption as well as presenting risks in a rational way. Mr. Seeb once again raised the issue of funding – reiterating that much of the funding for cannabis public education programs came from the marijuana cash tax fund – something that the natural medicine program does not yet have.
Allison Robinette then gave a quick review of identified audiences including the general public, interested participants, parents, potential applicants and licensees, schools, teachers, and educators.
The Department provided the following questions to guide discussion:
- Do we have the appropriate and encompassing scope of target audiences for public education campaigns?
- Which audiences should be prioritized for public education campaign development and launch?
- What types of resources are most effective in public education campaigns?
- What, if any, perceived gaps exist in public education regarding Natural Medicines?
Nine community stakeholders shared their thoughts on a range of topics. An animal advocate shared that animal owners should be educated on the issue of pets consenting to their owner consuming natural medicine, as well as a need for veterinarians and human therapists to be educated on the effects natural medicines may have on animals.
Additional audiences identified included people seeking natural medicine under personal use protections for mental health or addiction; veterans dealing with trauma; mental health providers whose patients may be using natural medicine outside of their supervision; healthcare providers; clinical pharmacists; DEI experts; rural community leaders; existing professional networks; and Indigenous communities.
Other considerations raised were the need for data collection, providing educational materials that are accessible to a diverse set of communities, school districts’ ability to dictate what is taught in schools, mitigating stigma associated with natural medicine broadly, communicating the safety profiles of natural medicines, and education on what qualifies someone as a trusted authority regarding natural medicine.
At the conclusion of the meeting DOR stated that a second listening session on this topic would be scheduled for the next round of sessions set to begin in November 2023.
Listening Session #4 – Testing Program Part 1
DOR representatives once again gave a very brief and high-level overview of SB23-290, quickly moving into statutory obligations relating to testing. Discussing overlap in statutory responsibility for testing between DOR and CDPHE, the Department identifies its initial role as setting the baseline requirements for licensing of a testing facility. This entails setting requirements for sampling, required tests, and testing frequency. CDPHE will be responsible under Title 25 for procedures that ensure correct labeling, product safety, acceptable levels of variance for concentration representations, product quarantine and notification procedures, and specific testing protocols. CDPHE views its initial role as establishing technical requirements to certify laboratories.
CHPHE representative Heather Krug gave an overview of general testing requirements under Colorado’s cannabis testing program which include 3rd-party testing at facilities licensed and certified by MED and CDPHE, test batch collection procedures and training, manifest and transfer protocols, photo documentation, and reporting in the inventory tracking system. Kyle Lambert discussed more technical aspects of cannabis testing. Those subjects included established limits for contaminant types, procedures in the case of failed tests, and test batch collection specifics.
Julia Bramante then gave an overview of Oregon’s psilocybin testing requirements which include species tests each month, potency tests for each batch of finished product, and solvent tests. Ms. Bramante also described procedures for failing tests.
The session then opened up for general discussion, during which 14 members of the public spoke regarding the following discussion questions:
- What testing categories are most critical for natural medicine? (e.g. heavy metals / elemental impurities, pesticides, microbial contaminants)
- What testing categories are specific to certain types of natural medicine? (e.g. psilocybe mushrooms)
- How frequently should the rules require licensees to conduct testing?
- At what points should testing occur? (e.g. prior to any transfer to a Facilitator or Healing Center, or on all final products)
- Who should be responsible for submitting samples for testing? (e.g. the facilitator, cultivation, manufacturer, healing center)
- Should DOR-licensed regulated marijuana testing facilities be permitted to test Natural Medicine?
Several people spoke to the need for testing psilocin content in addition to psilocybin content to ensure accurate dosing. One person suggested using “total psilocin content”, a number generated by converting psilocybin content to psilocin through a formula, as the most accurate way to convey potency of products. Several people also testified that water content is a significant factor in potency changes, encouraging testing for water content. A lab manager stated that heavy metals are the number one contaminant they have seen in their testing of mushrooms. Allowing labs to test in the personal use space was also encouraged by multiple people.
On species testing, several members of the public mentioned potential variations in experience between strains of P. cubensis. This was accompanied by suggestions to test for strain identification and additional alkaloids to allow facilitators to choose the most appropriate strain for a participant’s needs.
Other members expressed sentiments that the testing program should facilitate training and education for the Indigenous community; that the natural medicine program should take lessons from the cannabis program around regulatory difficulties and costs; the need to build a robust program that will be less susceptible to federal interference; the importance of including testing information in public education campaigns; the need to clearly differentiate whether weight requirements are by active compound or total weight for dosing and law enforcement purposes; and, that personal testing kits should be excluded from definitions of paraphernalia.
Listening Session #5 – Cultivation and Manufacturing Practices Part 1
Following formalities, Dominique Mendiola gave an overview of DOR’s statutory requirements under Title 44 regarding cultivation and manufacturing. Under the Department’s reading of the statute, their authority extends to
- Requirements and restrictions on types of products permitted
- Serving amounts and packaging (including the definition of a ‘microdose’)
- Health and safety standards
- Sanitary requirements
- Occupational health standards
- Recall plans and preventative controls
- Waste, disposal, & storage standards
- Sustainability
- Transportation of natural medicine
- Inventory, tracking, & manifest requirements
- Warning labels
- Production limits
Ms. Mendiola also pointed out statutory restrictions on natural medicine cultivation and manufacturing businesses. First, natural medicine products may not mimic a trademarked food product.1 Second, a cultivation or manufacturing facility may not be located within 1000 feet of a child care center, preschool, K-12 school, or residential child care facility.2
15 members of the public spoke in the 45 minutes of open discussion on the following questions:
- What traditional or historical cultivation practices can serve as a foundation for regulatory guardrails or requirements?
- What is the average weight of a single flush of psilocybin?
- What types of products should be permitted (or prohibited) within the regulated space?
- Do certain product types pose increased safety risks that need to be considered or addressed through regulations?
- What are the emerging product types and should those emerging products be subject to more stringent or additional requirements in order to be utilized in an administration session?
- If the Department has the authority to limit the number of cultivation and manufacturing licenses issued, should it?
Several members felt that average flush size was not a useful indicator to track, given the variation in potency and mushroom size by strain. At least two members encouraged DOR to limit natural medicine products to their natural form of whole or ground fruiting bodies, citing the fact that other alkaloids play an important role.
Several members of the public also discussed products that currently exist in the gray market, most commonly chocolate bars. Other products mentioned were teas, gummies, extracts, tinctures, capsules, and pressed tablets.
The issue of natural medicine’s susceptibility to degradation by water was mentioned multiple times in relation to manufacturing and packaging processes as well as regarding the need to test potency in the final forms of products to accurately account for degradation that may have occurred during manufacturing.
Other concerns raised by the community were the need for guardrails protecting smaller cultivators, the safety profile of manure as a cultivation substrate, drying methods, and the need for a transportation system which allows law enforcement to easily recognize a legal transfer.
At the close of the meeting, DOR indicated that the next round of Listening Sessions will begin in November and sessions will increase to 1.5-2 hours. In addition to building on some of the previous round, the next round will cover data collection, inventory tracking, package and labeling, businesses and licensing consideration
More information about the Department of Revenue’s Natural Medicine Division, including upcoming Listening Sessions can be found on the Department’s website. Parties interested in providing information to the Department are asked to reach out to the Natural Medicine Division’s Director of Policy & Regulatory Affairs, Allison Robinette, at allison.robinette@state.co.us.