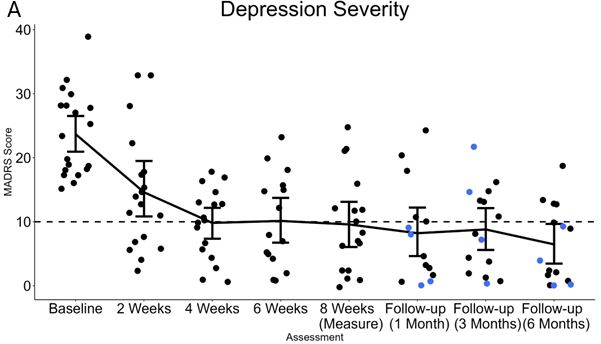
Vancouver, British Columbia –TheNewswire –29 October, 2024–MindBio Therapeutics Corp. (CSE: MBIO); (Frankfurt: WF6),(the “Company” or “MindBio”),is a leading clinical stage biopharmaceutical company in psychiatric medicine development using microdoses of psychedelic medicines to treat depressive disorders. The Company is delighted to report a significant and sustained antidepressant response in patient follow up 6 months post an 8-week treatment cycle with MB22001 in Phase 2A clinical trials.
Severity of Depression: Montgomery-Asberg Depression Rating Scale (MADRS)
MB22001 is MindBio’s lead candidate drug, a proprietary, self-titratable microdose of lysergic acid diethylamide which has been designed specifically for safe, take-home use. MindBio is the only organization in the world that is running multiple Phase 2B clinical trials with government approvals for take-home use and handling of a psychedelic medicine by trial patients on their own and out in the community. Patients self-administer the drug in microdoses at home, (the microdoses are sub-hallucinogenic), and patients are then able to get on with their day in the same way they would if they were taking any other medication.
MindBio’s thesis is that microdosing psychedelic medicines is a globally scalable, effective, affordable way to treat patients with depressive disorders. The Company currently has two Phase 2B clinical trials dosing and underway (a depression trial and a cancer trial) and those trials will run well into 2025.
The Phase 2a clinical trial demonstrated excellent safety, adherence and tolerance profile in doses tested. This was consistent with the Phase 1 trial results. The anti-depressant effects aresustained at 6 months post treatment with an impressive 72% reduction in depressive symptoms and 58% complete remission from depression. The findings augment the mounting evidence that MB22001 is a safe and effective drug for treating depression with a psychedelic medicine to patients out in the community.
The Company has also recently had a third Phase 2B trial in women’s health treating PMS (Pre-Menstrual Syndrome) and PMDD (Pre-Menstrual Dysphoric Disorder) approved for take-home dosing.
Justin Hanka, Chief Executive Officer of MindBio said, “We are delighted to discover that MB22001 has shown a sustained antidepressant response six months after cessation of treatment. This is good news for our current dosing in Phase 2B clinical trials underway and further supportive that we have developed a groundbreaking potential new treatment for depression. This data is a supportive big leap towards our commercialization objective”.
We invite you to join us in support of creating a brighter future for mental health.
Receive our latest updates here: https://www.mindbiotherapeutics.com/get-updates
Follow MindBio on LinkedIn: https://www.linkedin.com/company/mindbio-therapeutics/?viewAsMember=true
Follow CEO Justin Hanka on LinkedIn:https://www.linkedin.com/in/justinhanka/
For further information, please contact:
Justin Hanka, Chief Executive Officer
justin@mindbiotherapeutics.com
pr@hlthcommunications.com
About MindBio Therapeutics
MindBio is a leading biotech/biopharma company focused on creating novel and emerging treatments for mental health conditions and is conducting world first take-home Microdosing (MB22001) human clinical trials. MB22001 is MindBio’s lead candidate drug, a proprietary titratable form of Lysergic Acid Diethylamide (LSD) designed for take-home microdosing. MindBio is a leader in microdosing of psychedelic medicines and is advancing its drug and technology protocols through clinical trials. MindBio has developed a multi-disciplinary platform for developing treatments and is involved in psychedelic medicine development and digitaltherapeutics, has completed Phase 1 clinical trials in 80 healthy participants and has completed a Phase 2a clinical trial in patients with Major Depressive Disorder, both trials with positive top line data reported. Currently underway are two Phase 2B trials, one in cancer patients experiencing existential distress and another in patients with Major Depressive Disorder. The Company is also approved for multiple Phase 1/Phase 2B trials in women’s health. MindBio invests in research that forms the basis for developing novel and clinically proven treatments including digital technologies and interventions to treat debilitating health conditions such as depression, anxiety and other related mental health conditions.