Incannex Healthcare Limited (Nasdaq: IXHL / ASX: IHL), a clinical-stage pharmaceutical company developing unique medicinal cannabinoid pharmaceutical products and psychedelic medicine therapies for unmet medical needs, is pleased to announce that it has received final results from the Phase 1 clinical trial undertaken to assess pharmacokinetics and safety of the anti-inflammatory drug IHL-675A.
IHL-675A is a combination cannabinoid drug comprising cannabidiol (‘CBD’) and hydroxychloroquine (‘HCQ’) in a fixed dose combination. IHL-675A was observed to outperform either CBD and HCQ in various pre-clinical models of inflammation, including in vivo models of rheumatoid arthritis, inflammatory bowel disease and lung inflammation. Synergistic anti-inflammatory activity of CBD and HCQ was observed in these distinct pre-clinical studies and was evidence to support the Company’s international patent application over the drug.
The Phase 1 trial measured the safety, tolerability, and pharmacokinetic profiles of IHL-675A compared to the reference listed drugs, Epidiolex (CBD) and Plaquenil (HCQ). Three cohorts of 12 participants (n = 36) received either IHL-675A, CBD or HCQ and the clinical assessments were identical across the three arms of the trial. Participants were monitored for adverse events and had blood samples collected for pharmacokinetic analysis over a four-week period. The trial was conducted by CMAX Clinical Research in Adelaide, South Australia and managed by Avance Clinical.
Safety and Tolerability Results
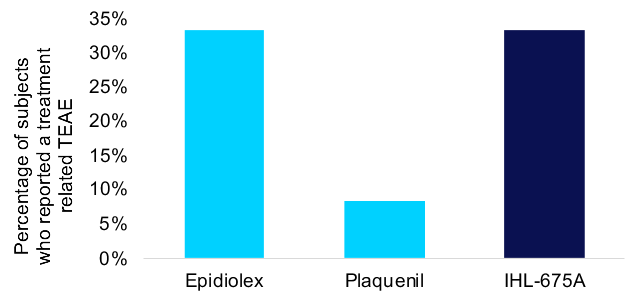
IHL-675A was well tolerated, with no adverse events of concern and no serious adverse events reported. The same number of treatment related treatment emergent adverse events (TEAEs) were reported for IHL-675A as for Epidiolex. Treatment-related TEAEs included abdominal pain, dizziness, fatigue, frequent bowel movements, headache and somnolence. All TEAEs were minor with the exception of one incidence of moderate severity abdominal cramps which resolved soon after on set.
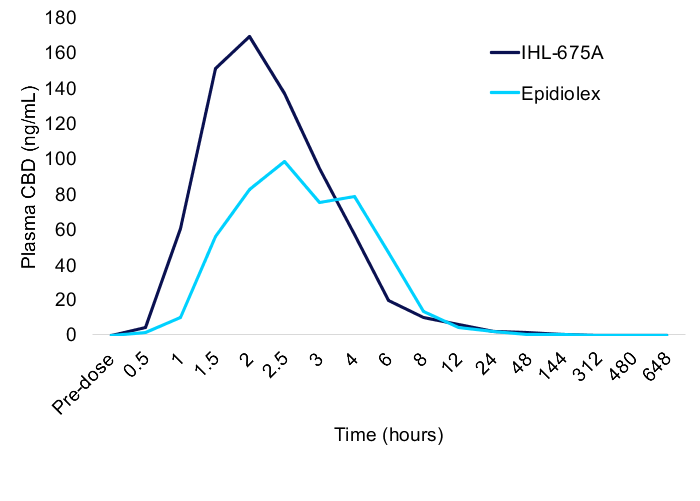
CBD Pharmacokinetic Results
Comparison of the average pharmacokinetics of CBD in participants administered IHL-675A compared to those administered Epidiolex revealed that the CBD was taken up from IHL-675A more quickly and reached a higher maximum concentration than from Epidiolex. The average maximum concentration (Cmax) of CBD from IHL-675A was 1.57 times higher than for Epidiolex. The time to reach the maximum concentration (Tmax) was 26% faster for IHL-675A than Epidiolex. CBD administered in IHL-675A was also cleared more quickly than Epidiolex. The half-life (t1/2) of CBD from IHL-675A was 13% faster than Epidiolex. The total exposure (AUCinf) was similar for CBD administered as IHL-675A and Epidiolex. These patterns are trends at this point (p >0.05). Similar results were observed for CBD metabolites 7-COOH-CBD and 7-OH-CBD. Data is presented in the appendix.
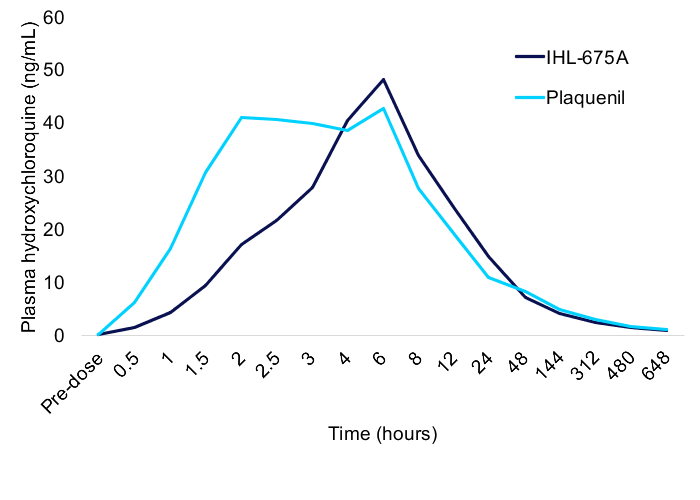
Hydroxychloroquine Pharmacokinetic Results
Comparison of the average pharmacokinetics of hydroxychloroquine in participants administered IHL-675A compared to those administered Plaquenil revealed that hydroxychloroquine was taken up more slowly from IHL-675A than from Plaquenil but the two drugs had a similar maximum plasma concentration. The time to reach the maximum concentration (Tmax) for HCQ administered as IHL-675A was 46% slower than for Plaquenil.
The hydroxychloroquine clearance and total exposure was similar for the two drugs. These patterns are trends at this point (p >0.05). Plasma concentrations of hydroxychloroquine metabolites desethylhydroxychloroquine, bisdesethylhydroxychloroquine and desethylchloroquine were detected only at low levels (<2 ng/mL) at all points in the study. Data is presented in the appendix.
Interpretation of the Results
IHL-675A is well tolerated in healthy volunteers. Adverse events for IHL-675A were consistent with what was observed, and has been publicly reported, for Epidiolex and Plaquenil. Both active pharmaceutical ingredients, CBD and HCQ, are absorbed from IHL-675A. Trends in PK profiles indicate that the uptake of CBD may be more rapid for IHL-675A than Epidiolex and uptake of HCQ may be slower for IHL-675A than Plaquenil. This could be advantageous for IHL-675A. CBD provides immediate relief for inflammation and pain whereas HCQ is a slower acting molecule and provides extended relief.
The safety and pharmacokinetic data from this Phase 1 clinical trial in healthy volunteers adds to the company’s confidence in proceeding with assessment of IHL-675A in patients with inflammatory diseases.
Incannex Chief Scientific Officer Dr Mark Bleackley said: “The results from the Phase 1 trial are a critical milestone in the development of IHL-675A. We would like to acknowledge the contributions of Avance and CMAX on conducting this trial and thank the volunteers who participated in the study. Data on the safety and pharmacokinetics of the drug combination are essential for supporting to administration of the drug to rheumatoid arthritis patients on a daily basis over an extended period in the Phase 2 trial. We are excited to better understand the therapeutic potential of IHL-675A as the development pipeline continues to progress.”
Next Steps – Phase 2 Trial for IHL-675A in Patients with Rheumatoid Arthritis
As HCQ is already approved for treatment of rheumatoid arthritis, it is the first indication for which IHL-675A will be assessed in a Phase 2 clinical trial. The trial will include 120 participants who meet the eligibility criteria. Participants will be randomised to one of 4 arms: either IHL-675A, CBD alone, HCQ alone or placebo. The primary endpoint for the study is pain and function relative to baseline determined via the score on the RAPID3 assessment at 24 weeks. The trial will be conducted in Australia and will update the ASX and Nasdaq once approval to conduct the trial is received from a human research ethics committee (HREC).
Trial designs for Phase 2 studies in patients with inflammatory bowel disease and lung inflammation are also being developed. The treatment of these three indications has a combined global annual market size of exceeding US$125B per annum1.
In addition, Incannex has filed a request for pre-IND meeting on the development of IHL-675A for treatment of arthritis with the FDA. Following the pre-IND meeting, the Company intends to open an IND in parallel with the Australian Phase 2 study, allowing for the conduct of trials in the US if the Australian study continues to support the therapeutic potential of IHL-675A in patients with arthritis.
In March of 2021, Incannex announced results from an in vivo model of rheumatoid arthritis where IHL-675A was observed to benefit the treatment of rheumatoid arthritis in mice greater than that of CBD or HCQ alone. In fact, low dose IHL-675A was 1.06x to 3.52x more effective at reducing arthritis across multiple disease assessments including clinical score, paw volume, pannus score, total histology score and serum cytokine levels as the standard dose of HCQ. HCQ is widely used for treatment of rheumatoid arthritis in the form of hydroxychloroquine sulphate; marketed as Plaquenil. An improvement to patient wellbeing achieved by IHL-675A would potentially open a major economic opportunity for Incannex in arthritis, being one of the three indications being pursued.
“A large sub-group of people with rheumatoid arthritis are using hydroxychloroquine or cannabidiol to help to alleviate their symptoms. We are delighted with the safety data and with the unique pharmacokinetic characteristics of IHL-675A that may be beneficial for patients with rheumatoid arthritis. By undertaking pivotal clinical studies over IHL-675A, we intend to disrupt the markets for both CBD and hydroxychloroquine as they currently pertain to the treatment of rheumatoid arthritis.”
Incannex CEO and Managing Director, Mr Joel Latham
About Incannex Healthcare Limited
Incannex is a clinical stage pharmaceutical development company that is developing unique medicinal cannabis pharmaceutical products and psychedelic medicine therapies for the treatment of obstructive sleep apnoea (OSA), traumatic brain injury (TBI) and concussion, lung inflammation (ARDS, COPD, asthma, bronchitis), rheumatoid arthritis, inflammatory bowel disease, anxiety disorders, addiction disorders, and pain, among other indications.
U.S. FDA approval and registration, subject to ongoing clinical success, is being pursued for each drug and therapy under development. Each indication under investigation currently has no, or limited, existing registered pharmacotherapy (drug) treatments available to the public and represent major global economic opportunities to Incannex and its shareholders.
Incannex has a strong patent filing strategy in place as it develops its products and therapies in conjunction with its medical and scientific advisory board and partners. The Company holds 19 granted patents and 29 pending patents.