We greatly appreciate your patience and unwavering support as we navigate the intricate landscape of building and expanding our psychedelic supply enterprise.
Throughout 2023, our primary focus has remained centered in Princeton, British Columbia, Canada, operating under the regulatory oversight of Health Canada. Our dedicated efforts have revolved around the meticulous development of our psychedelic drug candidates with stringent adherence to Good Manufacturing Practices (GMP). Concurrently, we have been diligently safeguarding our intellectual property (IP) and working daily to secure essential supply contracts. Your support during this period has been invaluable.
Turning our attention to the upcoming year, 2024 promises an influx of significant developments. We are currently engaged in comprehensive stability studies for various drug candidates, encompassing Psilocybin, MDMA, and we eagerly anticipate the opportunity to share in-depth insights as we make further progress.
The past year we consciously favoured debt financing over the dilution of shareholder equity given the market conditions. This approach serves as a prudent means of non-dilutive access to capital while we work to make our MDMA and Psilocybin drug candidates more accessible.
All of our founders hold meaningful financial positions in Optimi and share a profound commitment to the overall success of our business. Our collective objective remains the enhancement of shareholder value, and we are resolute in this pursuit to create sustainable, enduring value. As the psychedelic sector matures, clinical trials advance, and regulatory frameworks evolve globally, our state-of-the-art facility stands ready to meet the demand.
It is noteworthy that Optimi maintains its own licenses for the production of our psychedelics in-house. This strategic positioning enables us to provide competitively priced products within the GMP psychedelics market.
Going forward, Optimi will continue to be capitalized and supported by Founders who are deeply committed to our long-term goals. For those who share in our journey with patience, we remain confident that your commitment will yield rewarding results as the market opens up in Canada.
In fact, we are seeing momentum in Canada at the federal level with last week’s release of the Senate Subcommittee on Veterans Affairs report titled, “Granting equitable access to psychedelic-assisted therapies.” If you haven’t had a chance to read the report, we encourage you to take a few minutes to read it here.
In Australia, we are actively addressing the import process and collaborating closely with Mind Medicine Australia to navigate the intricacies related to the Authorised Prescribers Scheme. Currently, only individual Australian psychiatrists are eligible to become Authorised Prescribers. To become an Authorised Prescriber they will need to follow a two-step process:
- Obtain human research ethics committee (HREC) approval; and,
- Approval of an appropriate clinical protocol for the delivery of psychedelic-assisted psychotherapy.
After the psychiatrist successfully completes these steps and obtains approval from Australia’s regulatory authority, the Therapeutic Goods Administration – TGA, they can proceed to request an import permit for Optimi drug candidates that include MDMA or psilocybin. The approval for this import permit may take up to 45 days, following which Optimi will initiate the shipment of the product to Australia. Our commitment is focused on rapid progress, emphasizing swift advancements in this process.
With regard to our quality control efforts, we are meticulously testing each batch and preparing documentation for forthcoming Certificates of Analysis (COAs) for psilocybin, psilocybin extracts, and MDMA. A rigorous validation process, conducted in collaboration with a reputable third-party laboratory, underpins our commitment to transparency and data integrity. It will also make us the first publicly traded psychedelics company in Canada with full control of its scalable product from end-to-end.
These endeavors unfold daily at our on-site facility in Princeton, British Columbia, Canada, all while we uphold strict compliance with Health Canada’s regulations and safeguard the sanctity of our intellectual property. We are actively engaged in constructive dialogues with Health Canada with the aim of exploring potential amendments to our licenses for greater abilities on-site. Rest assured that we will promptly relay any official updates on this matter. We are committed to providing you with a detailed view of our progress and will do so respecting Health Canada regulations of what we can share.
On the Nutraceutical side of the business, we are starting to see some real traction in the Canadian Marketplace through retail sales in stores and through Amazon.ca as we set new sales records each month.
The Optimi Protein Powder, Optimi Formulation and Mindful are by far our best sellers with very high repeat sales. We are preparing the market for the launch of our Chocolate Protein Powder in the new year, and we are working hard with our broker and distributor to open new doors to drive more supply.
MEET DR. PRESTON A. CHASE
Last week, we announced the hiring of Dr. Preston A. Chase as our new Chief Science Officer. Dr. Chase is a distinguished Research & Development (“R&D”) chemist and seasoned business professional with an impressive academic and industrial track record spanning over 25 years. His extensive experience includes pioneering the development and commercialization of innovative products and processes for both multinational corporations and startup ventures.
He holds a BSc from the University of Victoria and a PhD from the University of Calgary, and a solid foundation in catalysis, synthesis, and analytical chemistry. With over 30 peer-reviewed scientific publications and numerous awarded and pending patents, he brings a wealth of research expertise and a strong history of commercial success to the Optimi Health team.
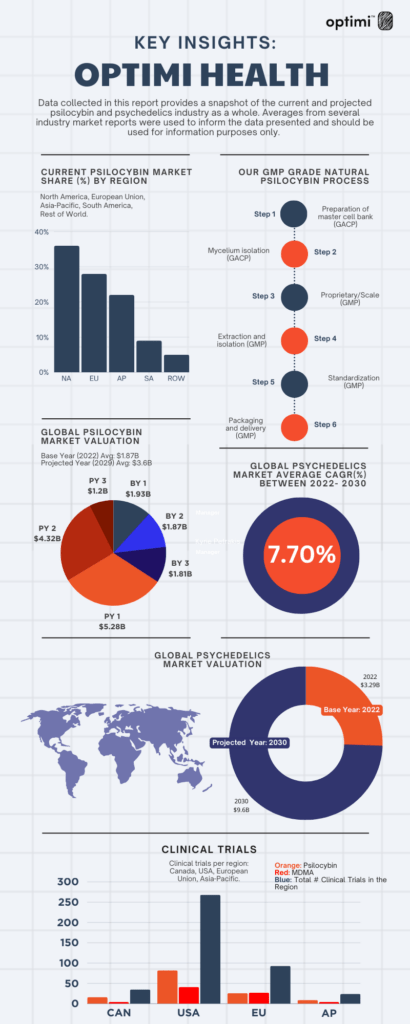
Annually, market research firms release Global Psychedelic Market Reports (GPMR), which encompass growth forecasts derived from a blend of qualitative and quantitative data originating from both public and private entities within the psychedelics industry. These foundational reports offer a comprehensive overview of the sector, encompassing aspects such as regulatory landscapes and significant macro-level developments, presenting an economic snapshot of the industry’s present position.
We recently took a deeper dive into the data and would like to provide our investors with some key insights. The data presented should be used for information purposes only and can be found here.
Thank you again for your support and trust in Optimi Health. We are committed to delivering on our mission and creating value for all our shareholders.
ABOUT OPTIMI
(CSE: OPTI) (OTCQX: OPTHF) (FRA: 8BN)
FOR INTERVIEW REQUESTS OR FURTHER INFORMATION PLEASE CONTACT:
Optimi Health Corp. an end-to-end drug researcher and formulator licensed by Health Canada to produce and supply, for clinical research purposes, psychedelic substances such as 3,4-Methylenedioxymethamphetamine (“MDMA”), natural GMP-grade psilocybin, as well as functional mushrooms that focus on the health and wellness markets. Built with the purpose of producing scalable psychedelic formulations for transformational human experiences, the Company’s goal is to be the number one trusted, compassionate supplier of safe drug products throughout the world. Optimi’s products are grown and manufactured at its two facilities comprising a total of 20,000 square feet in Princeton, British Columbia.